-
Physicians debate the comparative benefits of smart insulin delivery and pancreatic cell transplant
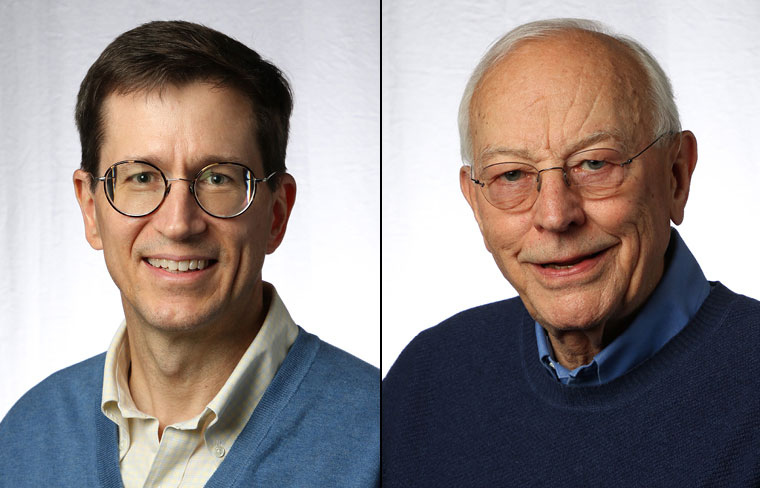
The debate continues over using beta-cell replacement or automated insulin delivery in the treatment of patients with type 1 diabetes. Michael R. Rickels, MD, MS, presented support for the biological solution provided by islet transplantation, while Bruce A. Buckingham, MD, presented data supporting the artificial pancreas.
-
Experts discuss the physiology of exercise and clinical management of athletes with type 1 diabetes
Michael Riddell, PhD, and Lori M. Laffel, MD, MPH, provided insight into the unique needs of athletes—children and adults—who live with type 1 diabetes. Different forms of exercise affect glycemia differently, making it important for the type, duration, and intensity of exercise to be factored into the athlete’s care plan.
-
ICYMI Presenter Profile: A Long-Acting Glucose-Dependent Insulinotropic Polypeptide Receptor Agonist Shows Weight Loss without Nausea or Vomiting
Filip K. Knop, MD, PhD, previews a presentation at the 83rd Scientific Sessions. Registered participants can view select recordings, including of this session, on the meeting’s virtual platform through August 28.
-
Time in range becomes an increasingly important diabetes target
A1C has long been the gold standard for assessing glycemic control in diabetes over time, but continuous glucose monitoring (CGM) metrics such as time in range (TIR) are increasingly becoming popular because they represent real-time results. Revital Nimri, MD, and other experts discussed the role of TIR and other CGM metrics that use the most…
-
Panelists look at impact of SGLT2 inhibitors, GLP-1 receptor agonists in CVD and diabetes
Growing evidence indicates SGLT2 inhibitors and GLP-1 receptor agonists have beneficial effects not only in patients with type 2 diabetes but also those at risk for cardiovascular disease. Physicians and scientists, including Darren K. McGuire, MD, MHSc, presented findings from trials that demonstrate positive clinical outcomes.
-
Symposium examines type 1 diabetes in other settings such as cancer immunotherapy and celiac disease
Cancer immunotherapy, monogenic primary immune deficiencies, and celiac disease may have pathologic links to type 1 diabetes. Four experts, including Arabella Young, PhD, discussed ways these conditions may be linked, and in some cases how they can be screened for together.
-
Session offers in-clinic tips for obtaining, interpreting diabetes device data
More than 3 million Americans use continuous glucose monitoring, which provides a plethora of useful data for clinicians. Obtaining and interpreting that data continues to present challenges. Rebecca Rick Longo, MSN, ACNP-BC, CDCES, offered solutions for efficiently downloading the data, and Cari Berget, MPH, RN, CDCES, explained a three-step process for reviewing and using the…
-
ICYMI Presenter Profile: Discussion on Diabetes Self-Management Education and Support (DSMES)
Clipper F. Young, PharmD, MPH, CDCES, BC-ADM, BCGP, APh, previews a Professional Interest Group discussion held at the 83rd Scientific Sessions. Registered participants can view select recordings, including of this session, on the meeting’s virtual platform through August 28.
-
Panel explores evolving impact of teplizumab on type 1 diabetes trials, treatment
Teplizumab became the first FDA-approved drug for the delay and prevention of type 1 diabetes, but it also comes with its own challenges. Presenters, including Kevan C. Herold, MD, shared the benefits of teplizumab along with some of the barriers faced for the research community, clinical practice, and patients.
-
DCCT/EDIC proves to be the most impactful research in diabetes history
Before DCCT/EDIC, most individuals with type 1 diabetes died in their late 40s. Four decades later, David M. Nathan, MD, Chair of the DCCT/EDIC Research Group, and other investigators explained how management of the disease has been transformed by the data gleaned from this groundbreaking research and, as a result, lifespans have been extended.